 Back to projects
Back to projects
Study of molecular mechanisms involved in the regulation of the nucleus size and a potential effect of the latter on chromatin organization in living cells
- Date: 2023
- Institution: Shenzhen Bay Laboratory, China
- Authors: Artem Efremov
- Aim of study:
In addition to regulation of cell migration, extracellular mechanical cues are also known to influence nuclear organization. Namely, it was previously shown that the elastic properties and topography of the substrate, as well as mechanical forces applied to living cells, have a significant impact on the epigenetic state of chromatin and gene transcription, frequently inducing cell differentiation or dedifferentiation processes. Previously, two potential mechanisms were proposed to explain these observations: 1) based on the Ca2+ influx through mechanosensitive Piezo1 channels that causes changes in the activity of enzymes involved in the epigenetic modification of chromatin, and 2) based on forcetransmitting links formed by nesprin-SUN protein complexes between the actin cytoskeleton and the nuclear envelope, which affect the shape of the nucleus and chromatin organization. However, most of the molecular processes underlying these two proposed pathways remain poorly understood.
To gain detailed insights into these mechanosensitive pathways, we use a combination of theoretical and experimental methods to predict and experimentally test the role of physical forces in shaping chromatin structure and its epigenetic state.
Specifically, over the past few years we have developed a general theoretical framework based on the polymer field theory describing the role of mechanical and thermodynamic forces in the regulation of the nuclear organization. This approach not only revealed the main molecular mechanisms underlying the famous correlation between cell and nucleus volumes, but also predicted that the cell nucleus functions as a piezoelectric element, changing its electrostatic potential in a size-dependent manner. This effect has been suggested to have a profound impact on stability of nucleosomes and other nucleoprotein complexes, revealing a previously unknown link between the nucleus size and chromatin structure.
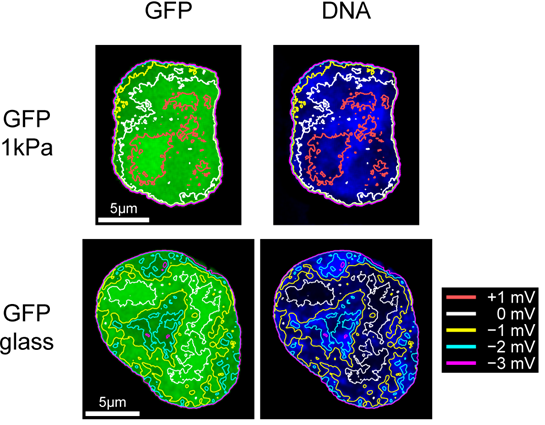
To test the above model prediction and find out whether the piezoelectric behaviour of the nucleus may potentially contribute to cell mechanosensing, we measured the distribution of small electrically charged fluorescent molecules in living cells grown on substrates of different elasticities. It was found that the nuclei of living cells do exhibit the predicted piezoelectric behaviour, which is sensitive to the substrate elasticity. Furthermore, by using GFP labelled Sox2 transcription factor together with FISH assay, it was shown that experimentally observed heterogeneity of the nuclear electrostatic potential may have a significant effect on the lncRNA-mediated localization of Sox2 on chromosomal DNA, thus revealing a new potential regulatory mechanism governed by electrostatic interactions between chromatin components and the nuclear electrostatic field. These results are currently being prepared for publication.
In the nearest future, we also plan to investigate molecular mechanisms underlying Ca2+-mediated and nesprin-mediated mechanotransduction pathways responsible for the regulation of the epigenetic state of chromatin. To this aim, we are going to use the micropipette aspiration assay to apply mechanical load to cells, as well as real-time Ca2+ level imaging with the help of GCaMP6f indicator, and visualization of the chromatin methylation state with the FRET biosensor developed by Dr. Qin Peng (SZBL, China). Combining these measurements with observations of nuclearcytoplasmic shuttling of methyltransferases and perturbing key elements of the mechanotransduction pathways with inhibitors or by conducting knockdown assays, we plan to gain a more detailed understanding of the molecular mechanisms underlying these mechanotransduction pathways and their contribution to changes in the epigenetic state of chromatin in response to extracellular mechanical cues.
