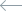 Back to projects
Back to projects
Investigation of mechanosensitive molecular pathways underlying activation of cell signaling cascades involved in the regulation of cell migration
- Date: 2022
- Institution: Shenzhen Bay Laboratory, China
- Authors: Artem Efremov
- Aim of study:
Filopodia play the central role in the mechanosensing of surrounding environment by living cells. However, the exact molecular mechanisms by which filopodia guide cell migration in a mechano-dependent manner remain poorly understood. Our previous experiments revealed that filopodia produce persistent Ca2+ signals in response to mechanical stretching due to force-induced Ca2+ influx through transmembrane L-type channels. This phenomenon was observed in several different cell lines, including human embryonic kidney cells (HEK-293), breast cancer epithelial cells (MCF-7) and metastatic melanoma cells (A2058), suggesting that it is based on rather universal molecular mechanisms. Furthermore, it was found that intra-filopodial Ca2+ influx often results in activation of the nearby cell cortex, which is frequently followed by lamellipodium propagation towards the stretched filopodium. Thus, force-induced Ca2+ signals generated by filopodia may potentially trigger a global cell response.
However, it is still unknown how L-type Ca2+ channels are able to sense the mechanical force applied to filopodia, and how the force-induced intrafilopodial Ca2+ signals results in activation of lamellipodium propagation in the direction of the pulled filopodium. Previous studies suggest that Filamin A could potentially play an important role in this process by connecting L-type Ca2+ channels to the force-bearing actin cytoskeleton. Specifically, it has been shown that depletion of Filamin A leads to dramatic decrease of the pressure-dependent Ca2+ influx into arterial myocytes through L-type channels, indicating a potential role of the cell cytoskeleton in its regulation. Yet, it is not known whether this mechanism is used by filopodia in their mechanosensory function or not.
To find answer to this question our research team has created a Filamin A KO HEK-293 cell line. Using it, we plan to check if the force-induced Ca2+ influx in mechanically stretched filopodia of such cells is significantly reduced in comparison to WT HEK-293 cells with the help of previously developed optical tweezers assay. Several preliminary experiments performed to date suggest that Filamin A is indeed essential for generation of intrafilopodial Ca2+ signals, and thus Filamin Amediated force-transmission from the actin cytoskeleton to L-type Ca2+ channels may be one of the main mechanisms involved in mechanodependent Ca2+ signalling of filopodia that guides cell migration.
As for the second question regarding the role of intrafilopodial Ca2+ signals in activation of the lamellipodium propagation, it is known that such processes are controlled by small Rho GTPases, which are the key elements of the cell signalling network involved in the regulation of cell migration. Thus, one might expect that the intrafilopodial Ca2+ influx may potentially activate some of these GTPases.
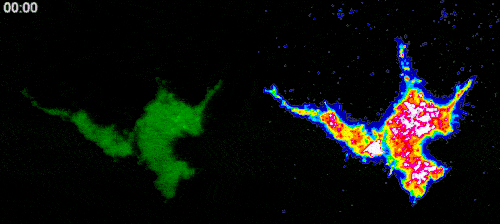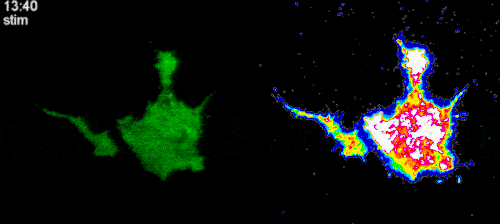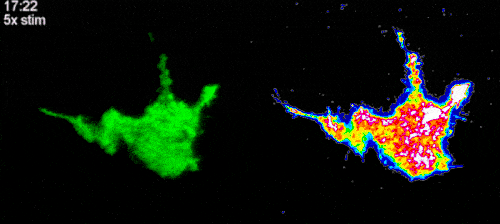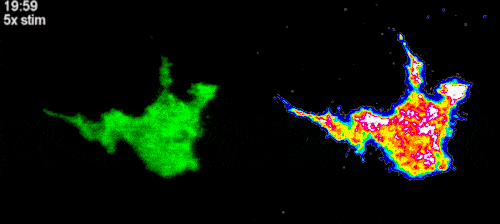
To test this hypothesis, we established a cell line expressing optogenetic Ca2+ channels, as well as Ca2+ indicator (GCaMP6f). By transfecting these cells with RhoA, Rac1 and Cdc42 biosensors and locally activating Ca2+ channels with light, we plan to systematically investigate whether local Ca2+ influx can cause activation of Rho GTPases. After selecting the potential GTPase candidates, we will use optical tweezers to stretch filopodia in cells expressing Ca2+ indicator and biosensors to the selected Rho GTPases to further reveal a potential role of the force-induced intrafilopodial Ca2+ influx in activation of these signalling proteins. This study is carried out in collaboration with the laboratory of Dr. Heath Johnson from the University of Hong Kong.
To gain more detailed insights into the potential crosstalk between mechanosensitive pathways and small Rho GTPases, we plan to investigate their role in the regulation of mechanical forces generated by epithelial cells in zebrafish embryos, which provide innate immune response by phagocyting apoptotic cells during the embryo development process. To this aim, we established in our laboratory a microparticle traction force microscopy, which we are going to combine with measurements of the elastic properties of apoptotic embryonic cells by AFM and/or optical tweezers in order to assess the forces generated during their phagocytosis by the epithelial cells. Then, using inhibitors or dominant negative mutants of Rho GTPases, we aim to investigate the role of these signalling molecules in the regulation of the phagocytosis by zebrafish embryo epithelial cells. This work is done in collaboration with the research group of Dr. Hoijman Esteban from University of Barcelona.
